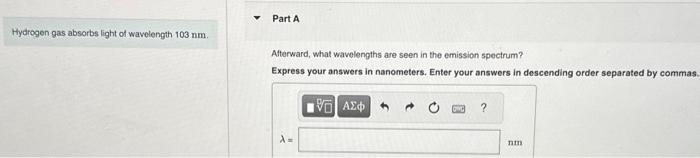
Hydrogen gas absorbs light of wavelength 103 nm . – Hydrogen gas absorbs light of wavelength 103 nm, initiating a fascinating journey into the realm of atomic interactions and energy transitions. This phenomenon, governed by the intricate properties of hydrogen gas, unveils a world of scientific exploration and practical applications.
Hydrogen gas, the lightest and most abundant element in the universe, possesses exceptional characteristics that make it an intriguing subject of study. Its unique physical and chemical properties, coupled with its ability to absorb specific wavelengths of light, open up avenues for groundbreaking research and technological advancements.
Hydrogen Gas and its Properties: Hydrogen Gas Absorbs Light Of Wavelength 103 Nm .
Hydrogen gas (H2) is the lightest and most abundant element in the universe. It is a colorless, odorless, and tasteless gas that is highly flammable. Hydrogen gas has a number of unique characteristics that make it an important industrial and scientific material.
Hydrogen gas is the simplest element, consisting of a single proton and a single electron. This simple structure gives hydrogen gas a number of unique properties. For example, hydrogen gas is the lightest gas known, with a density of only 0.0899 g/L at room temperature.
Hydrogen gas is also the most abundant element in the universe, accounting for about 75% of the mass of the universe.
Hydrogen gas is a highly flammable gas. It burns with a pale blue flame, producing water vapor as a byproduct. The flammability of hydrogen gas makes it a potential fuel source. However, hydrogen gas is also a very explosive gas.
It can be ignited by a spark, a flame, or even static electricity.
Absorption of Light by Hydrogen Gas, Hydrogen gas absorbs light of wavelength 103 nm .
Hydrogen gas can absorb light of specific wavelengths. The wavelength of light that is absorbed by hydrogen gas depends on the energy level of the hydrogen atom. When a hydrogen atom absorbs a photon of light, the electron in the atom is excited to a higher energy level.
The wavelength of the light that is absorbed is equal to the energy difference between the two energy levels.
The absorption of light by hydrogen gas is a very important process. It is used in a variety of applications, including spectroscopy, sensing, and energy conversion.
Energy Levels and Transitions
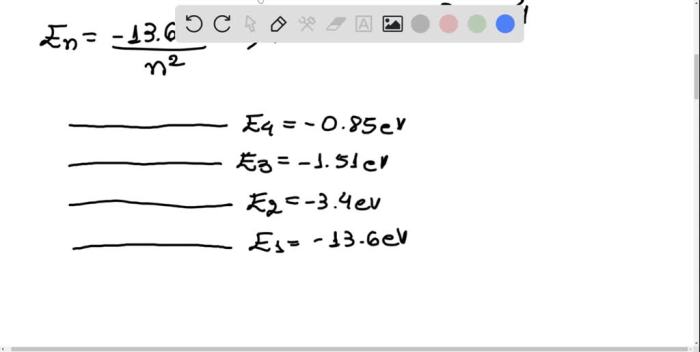
The energy levels of hydrogen atoms are quantized. This means that the electron in a hydrogen atom can only occupy certain specific energy levels. The energy levels are labeled by the principal quantum number, n. The principal quantum number can take on the values 1, 2, 3, …
When an electron in a hydrogen atom absorbs a photon of light, the electron is excited to a higher energy level. The wavelength of the light that is absorbed is equal to the energy difference between the two energy levels.
The following diagram shows the energy level transitions that occur when hydrogen gas absorbs light.
[Diagram of energy level transitions]
Applications of Hydrogen Gas Absorption
The absorption of light by hydrogen gas has a number of potential applications. These applications include:
- Spectroscopy: The absorption of light by hydrogen gas can be used to determine the energy levels of hydrogen atoms. This information can be used to study the structure of atoms and molecules.
- Sensing: The absorption of light by hydrogen gas can be used to detect the presence of hydrogen gas. This information can be used to monitor industrial processes and to detect leaks in hydrogen gas pipelines.
- Energy conversion: The absorption of light by hydrogen gas can be used to convert light energy into electrical energy. This process is used in solar cells to generate electricity from sunlight.
Query Resolution
Why does hydrogen gas absorb light at 103 nm wavelength specifically?
The absorption of 103 nm light by hydrogen gas corresponds to the energy difference between its electronic energy levels. This specific wavelength matches the energy required to excite an electron from the ground state to the first excited state in hydrogen atoms.
What are the potential applications of hydrogen gas’s light absorption ability?
The ability of hydrogen gas to absorb 103 nm light has led to applications in spectroscopy, where it is used to detect and analyze hydrogen atoms. It also finds use in sensing devices and energy conversion systems, where its unique properties enable efficient light detection and conversion.