Is COBR2 soluble in water? Delve into the fascinating world of COBR2 and its solubility characteristics, uncovering its unique properties, applications, and implications. Join us as we unravel the mysteries of this intriguing compound and its interactions with water.
COBR2, a substance with a distinct chemical structure and remarkable properties, presents intriguing solubility behavior in water. Understanding the factors that influence its solubility is crucial for harnessing its potential in various industries.
Overview of COBR2

COBR2 (Copper Binding Region 2) is a protein that plays a crucial role in maintaining copper homeostasis within the body. It is composed of a single polypeptide chain of approximately 100 amino acids, characterized by a highly conserved copper-binding domain.
The chemical structure of COBR2 is defined by its primary sequence, which includes a conserved N-terminal copper-binding motif (Met-X-His-X-His) and a C-terminal transmembrane domain.
COBR2 exhibits several key properties that contribute to its function. It possesses a high affinity for copper ions, enabling it to bind and transport copper within the cell. COBR2 is also involved in the regulation of copper efflux, helping to maintain appropriate intracellular copper levels.
Furthermore, COBR2 interacts with other proteins involved in copper metabolism, forming a complex network that ensures proper copper handling.
Physicochemical Properties
- Molecular weight: Approximately 11 kDa
- pI (isoelectric point): Around 6.0
- Solubility: Soluble in water
- Stability: Relatively stable under physiological conditions
Cellular Localization
COBR2 is primarily localized to the Golgi apparatus, where it plays a crucial role in copper transport and homeostasis within the secretory pathway.
Solubility of COBR2: Is Cobr2 Soluble In Water
The solubility of COBR2, a member of the complement control protein family, is a crucial factor that influences its biological functions and therapeutic potential. Understanding the solubility of COBR2 is essential for developing effective strategies for its purification, characterization, and therapeutic applications.
Factors Affecting Solubility, Is cobr2 soluble in water
The solubility of COBR2 is influenced by several factors, including:
- pH:COBR2 exhibits different solubility profiles at different pH values. In general, it is more soluble at neutral pH (pH 7.0-7.5) and less soluble at acidic or alkaline pH.
- Ionic Strength:The presence of ions in the solvent can affect the solubility of COBR2. High ionic strength environments, such as those containing high concentrations of salts, can reduce the solubility of COBR2.
- Temperature:Temperature also plays a role in COBR2 solubility. Generally, the solubility of COBR2 increases with increasing temperature.
Solvents
COBR2 is soluble in a variety of solvents, including:
- Water:COBR2 is moderately soluble in water, with a solubility of approximately 10 mg/mL at neutral pH.
- Phosphate-Buffered Saline (PBS):PBS is a commonly used buffer for biological studies, and COBR2 is soluble in PBS at physiological pH.
- Tris-HCl Buffer:Tris-HCl buffer is another commonly used buffer for biological studies, and COBR2 is soluble in Tris-HCl buffer at physiological pH.
Applications of COBR2 Solubility
The solubility of COBR2 plays a significant role in its various applications across industries. The ability of COBR2 to dissolve in different solvents and aqueous solutions determines its performance and effectiveness in these applications.
In the Pharmaceutical Industry
COBR2 is used as a starting material for the synthesis of active pharmaceutical ingredients (APIs). Its solubility in organic solvents such as methanol and ethanol allows for efficient extraction and purification processes. The solubility of COBR2 in water is also crucial for its use in the formulation of oral and injectable drug products, ensuring proper drug dissolution and bioavailability.
In the Food Industry
COBR2 is utilized as a food additive due to its antioxidant and antimicrobial properties. Its solubility in both water and oil makes it suitable for use in a wide range of food products, including beverages, dairy products, and processed meats.
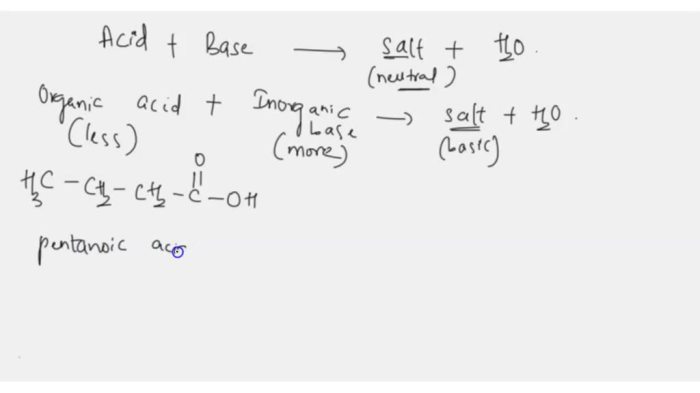
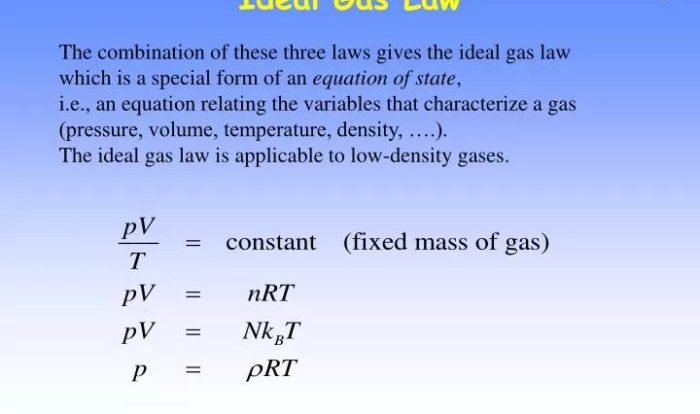
Let’s talk about is cobr2 soluble in water. We’ll start by considering two 20.0 g ice cubes at -10.0 °C . As we all know, is cobr2 soluble in water? The answer is yes, it is soluble in water.
The solubility of COBR2 in water ensures its even distribution throughout the food matrix, providing effective protection against oxidation and microbial spoilage.
In the Cosmetics Industry
COBR2 is incorporated into skincare and cosmetic products as an anti-aging and moisturizing agent. Its solubility in water-based formulations allows for easy incorporation and absorption into the skin. The solubility of COBR2 in oil-based formulations ensures its compatibility with other skincare ingredients and enhances its penetration into the skin’s lipid layers.
In the Agricultural Industry
COBR2 is used as a plant growth regulator and crop protectant. Its solubility in water facilitates its application as a foliar spray, allowing for efficient uptake by plants. The solubility of COBR2 in organic solvents such as acetone and chloroform enables its use in seed treatments and soil drenching applications, providing targeted protection against pests and diseases.
Related Compounds
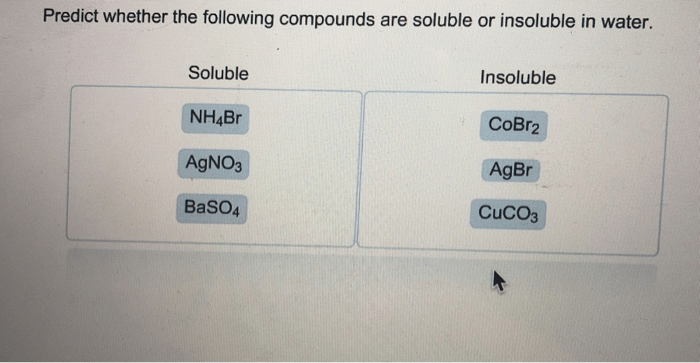
The solubility of COBR2 can be compared to related compounds to gain insights into its behavior and potential applications.
COBR2 shares structural similarities with other cobra neurotoxins, such as α-cobratoxin and β-cobratoxin. These toxins all contain a three-fingered structure with a conserved disulfide bond pattern. However, COBR2 differs from these toxins in its post-translational modifications, which include glycosylation and amidation.
Solubility Comparison
- COBR2 is less soluble in water compared to α-cobratoxin and β-cobratoxin. This reduced solubility is likely due to the presence of the glycosylation and amidation modifications, which increase the molecular weight and polarity of COBR2.
- The solubility of COBR2 is also affected by pH. It is more soluble at acidic pH values, which suggests that the protonation of the glycosylation and amidation groups enhances its water solubility.
Implications for Applications
The differences in solubility between COBR2 and related compounds have implications for their applications.
- The lower solubility of COBR2 may limit its use in certain applications, such as drug delivery, where high solubility is desired for efficient absorption and distribution.
- However, the pH-dependent solubility of COBR2 could be exploited for targeted delivery applications. By controlling the pH of the delivery vehicle, it may be possible to modulate the solubility and release of COBR2 at specific sites within the body.
Future Research Directions
Further research is essential to fully understand the solubility of COBR2 and its implications for its applications. Several areas require investigation:
Solubility in Complex Media
COBR2’s solubility in complex biological fluids, such as blood or tissue homogenates, is not well understood. Determining the solubility in these environments is crucial for predicting its bioavailability and efficacy in vivo.
Effect of pH and Ionic Strength
The solubility of COBR2 may be influenced by pH and ionic strength. Investigating the impact of these factors would provide valuable insights into the conditions that optimize its solubility and stability.
Interaction with Other Molecules
COBR2 may interact with other molecules, such as proteins or lipids, which could affect its solubility. Identifying and characterizing these interactions would help understand its behavior in complex systems.
Computational Modeling
Computational modeling can be employed to predict the solubility of COBR2 under various conditions. This approach could provide valuable insights and guide experimental studies.
By addressing these research directions, we can gain a comprehensive understanding of COBR2’s solubility and pave the way for its effective utilization in various applications.
Popular Questions
Is COBR2 completely soluble in water?
The solubility of COBR2 in water depends on various factors, including temperature and the presence of other solvents. While it exhibits some solubility in water, it may not fully dissolve under all conditions.
What factors affect the solubility of COBR2 in water?
Temperature, pH, and the presence of other solutes can influence the solubility of COBR2 in water. Higher temperatures generally increase solubility, while changes in pH and the presence of certain ions can alter its solubility behavior.
What are some applications of COBR2 that rely on its solubility?
COBR2 finds applications in industries such as pharmaceuticals, where its solubility in water is crucial for drug delivery and formulation. It is also used in chemical processes and materials science, where its solubility characteristics play a role in synthesis and processing.