The electron ionization mass spectrum of a hydrocarbon is shown, providing a wealth of information about its molecular structure and composition. This powerful analytical tool offers insights into the fragmentation patterns, molecular ion peak, base peak, isotopic peaks, and the presence of branching, ring structures, and functional groups within the hydrocarbon molecule.
By examining the mass-to-charge ratio of ions produced during electron ionization, scientists can deduce the molecular weight, identify structural features, and determine the presence of specific functional groups. This technique has become an indispensable tool in various fields, including organic chemistry, petroleum engineering, and forensic science.
Fragmentation Patterns
The electron ionization mass spectrum of hydrocarbons exhibits characteristic fragmentation patterns that provide valuable information about the molecular structure. Common fragmentation patterns include:
- Alpha-cleavage:Loss of a neutral hydrogen atom (H) from the carbon adjacent to the positively charged carbon ion, resulting in a fragment with a mass difference of 14.
- Beta-cleavage:Loss of a neutral alkyl group (R) from the carbon adjacent to the positively charged carbon ion, resulting in a fragment with a mass difference of 28.
- McLafferty rearrangement:Rearrangement of a protonated alkyl group (RCH 2+) to form an alkene and a carbocation, resulting in fragments with mass differences of 28 and 42.
- Retro-Diels-Alder reaction:Fragmentation of a cyclohexene ring into two smaller alkenes, resulting in fragments with mass differences of 82 and 96.
Specific examples of hydrocarbon fragmentation patterns include:
- Propane:M +=44, alpha-cleavage (CH 3+, m/z 15), beta-cleavage (C 2H 5+, m/z 29)
- Butane:M +=58, alpha-cleavage (CH 3+, m/z 15), beta-cleavage (C 3H 7+, m/z 43), McLafferty rearrangement (C 3H 5+, m/z 41)
- Cyclohexane:M +=84, retro-Diels-Alder reaction (C 4H 6+, m/z 54, C 2H 4+, m/z 28)
Molecular Ion Peak: The Electron Ionization Mass Spectrum Of A Hydrocarbon Is Shown
The molecular ion peak (M +) in the mass spectrum corresponds to the mass of the intact molecule. It is significant because it provides direct evidence of the molecular weight and elemental composition of the hydrocarbon.
To identify and interpret the molecular ion peak, the following steps are taken:
- Locate the peak with the highest mass-to-charge ratio (m/z) in the mass spectrum.
- Determine if the peak is a singly charged ion (z=1). This can be done by examining the isotopic peaks adjacent to the molecular ion peak.
- Compare the m/z value of the molecular ion peak with the expected molecular weight of the hydrocarbon based on its molecular formula.
For example, the molecular ion peak for butane (C 4H 10) would be observed at m/z 58.
Base Peak
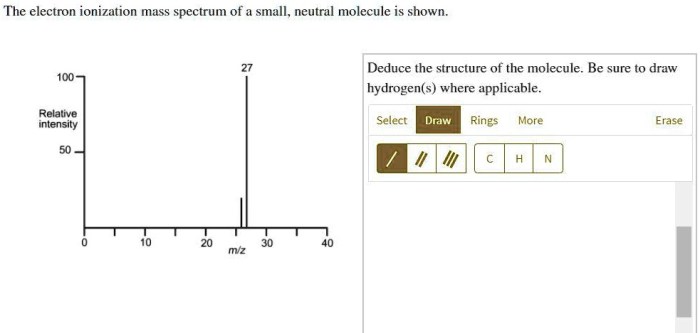
The base peak in the mass spectrum is the most intense peak, representing the most abundant fragment ion. It is important because it provides a reference point for interpreting the relative abundances of other fragment ions.
Factors that influence the base peak in the mass spectrum of hydrocarbons include:
- Fragmentation patterns:The base peak often corresponds to a fragment ion that is formed through a highly favorable fragmentation pathway.
- Molecular weight:Heavier hydrocarbons tend to have a higher base peak due to the increased number of possible fragmentation pathways.
- Branching and ring structures:Branching and ring structures can stabilize certain fragment ions, making them more abundant.
Isotopic Peaks
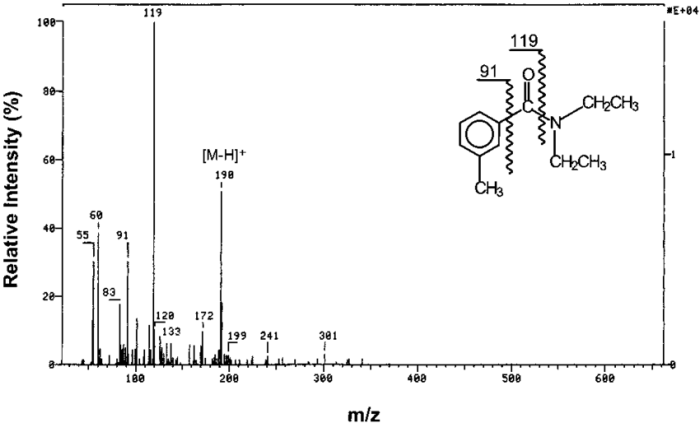
Isotopic peaks in the mass spectrum of hydrocarbons arise due to the presence of naturally occurring isotopes of carbon and hydrogen. The most common isotopes are 12C, 13C, 1H, and 2H (deuterium).
The pattern and abundance of isotopic peaks provide information about the elemental composition of the hydrocarbon. For example, the presence of a peak at m/z 59 in addition to the molecular ion peak at m/z 58 indicates the presence of 13C in the molecule.
The abundance of isotopic peaks can be predicted using the natural abundance of the isotopes. For example, the abundance ratio of 13C to 12C is approximately 1:100, so the intensity of the isotopic peak at m/z 59 would be approximately 1% of the intensity of the molecular ion peak at m/z 58.
Branching and Ring Structures
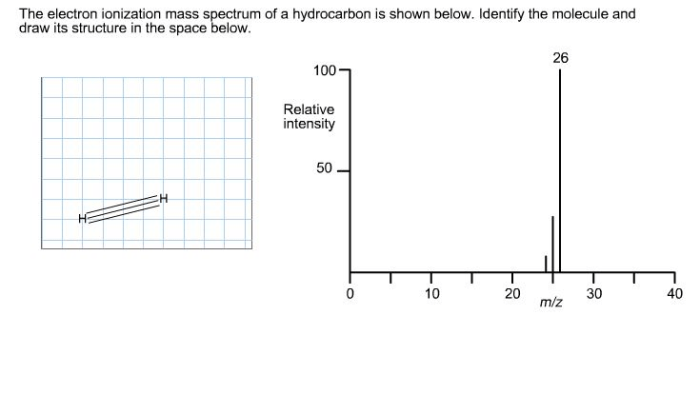
Branching and ring structures in hydrocarbons can affect the fragmentation patterns and mass spectra. Branching can stabilize certain fragment ions, making them more abundant. For example, branched alkanes often exhibit a more intense peak at m/z 43 (C 3H 7+) due to the increased stability of the tertiary carbocation formed in the beta-cleavage process.
Ring structures can also influence the mass spectra of hydrocarbons. Cyclic alkanes often exhibit a retro-Diels-Alder reaction, resulting in the formation of fragment ions with mass differences of 82 and 96. Additionally, ring structures can stabilize certain fragment ions through resonance, making them more abundant.
Functional Group Identification
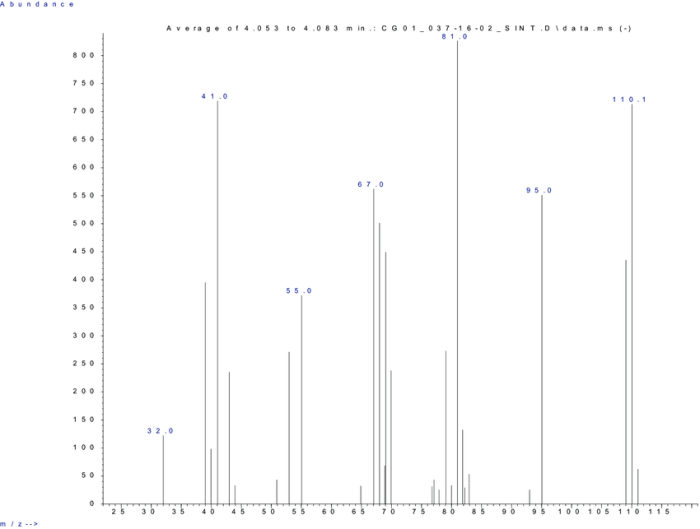
The presence of functional groups in hydrocarbons can be identified from the mass spectrum based on characteristic fragmentation patterns.
- Alcohols:Alcohols often exhibit a peak at m/z 31 (CH 3OH +) due to the loss of a water molecule (H 2O).
- Ketones:Ketones often exhibit a peak at m/z 43 (CH 3CO +) due to the loss of a methyl radical (CH 3•).
- Aldehydes:Aldehydes often exhibit a peak at m/z 29 (CHO +) due to the loss of a hydrogen radical (H •).
- Carboxylic acids:Carboxylic acids often exhibit a peak at m/z 45 (COOH +) due to the loss of a hydroxyl radical (OH •).
Answers to Common Questions
What is the significance of the molecular ion peak in the mass spectrum?
The molecular ion peak represents the intact parent molecule, providing information about its molecular weight. It is crucial for determining the empirical formula and molecular structure of the hydrocarbon.
How can we identify branching and ring structures from the mass spectrum?
Branching and ring structures result in characteristic fragmentation patterns. For example, branched hydrocarbons exhibit a higher abundance of low-mass fragment ions, while ring structures show more stable fragment ions with higher mass-to-charge ratios.
What role do functional groups play in the mass spectrum of hydrocarbons?
Functional groups introduce specific fragmentation patterns that can be used to identify their presence. For instance, the presence of an oxygen-containing functional group often leads to the formation of fragment ions with characteristic mass-to-charge ratios.