Dive into the realm of gases with Ideal Gas Law Gizmo Answers, an extraordinary resource that unveils the mysteries surrounding these elusive substances. This comprehensive guide delves into the fundamental principles of the ideal gas law, empowering you with a profound understanding of how pressure, volume, temperature, and the number of moles interact within an ideal gas.
The Ideal Gas Law Gizmo simulation serves as a captivating platform for experimentation, allowing you to manipulate variables and collect data with ease. Through a series of engaging experiments, you’ll unravel the intricate relationships between these properties, gaining valuable insights into the behavior of gases.
Ideal Gas Law Concepts
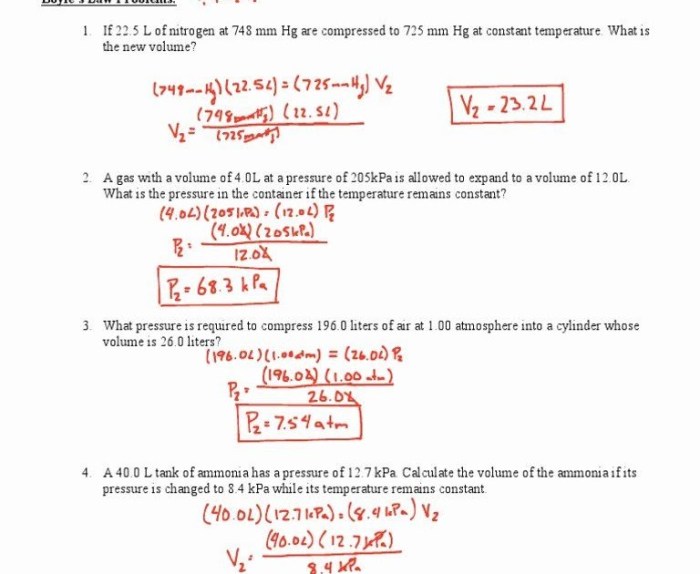
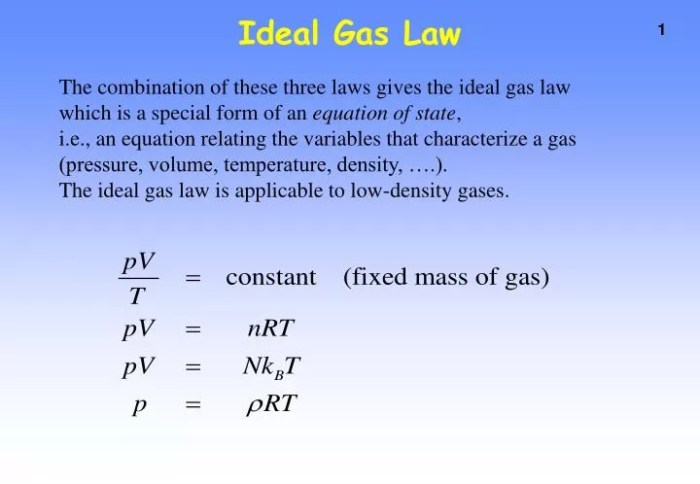
The ideal gas law, also known as the perfect gas law, is a mathematical equation that describes the relationship between pressure, volume, temperature, and the number of moles of a gas. It is a fundamental law in chemistry and physics, and it has a wide range of applications in various fields, including thermodynamics, engineering, and environmental science.
Relationship between Pressure, Volume, Temperature, and Number of Moles
The ideal gas law states that the pressure of a gas is directly proportional to its temperature and the number of moles of gas, and inversely proportional to its volume. This relationship can be expressed mathematically as:
PV = nRT
- P is the pressure of the gas in pascals (Pa)
- V is the volume of the gas in cubic meters (m³)
- n is the number of moles of gas in moles (mol)
- R is the ideal gas constant, which is equal to 8.314 J/mol·K
- T is the temperature of the gas in kelvins (K)
The ideal gas law can be used to solve a variety of problems involving gases, such as calculating the pressure, volume, temperature, or number of moles of a gas when the other three variables are known.
Gizmo Simulation Overview
The “Ideal Gas Law Gizmo” simulation is an interactive virtual environment designed to help students explore the behavior of gases under various conditions. It allows users to manipulate variables such as temperature, volume, pressure, and number of moles, and observe the corresponding changes in the gas’s properties.
The simulation features a container filled with gas molecules, represented as colored spheres. Users can adjust the temperature by dragging a slider, which changes the average kinetic energy of the molecules. The volume can be modified by dragging the edges of the container, which changes the space available for the molecules to move.
The pressure is displayed as a gauge on the side of the container, and it changes as the volume or temperature is adjusted.
Ideal gas law gizmo answers can be a valuable resource for understanding the behavior of gases. If you’re looking for more practice with gas laws, consider checking out the tamu chem 107 past exams . These exams offer a range of questions on gas laws, providing an excellent way to test your understanding and prepare for upcoming assessments.
By utilizing both gizmo answers and past exams, you can gain a comprehensive grasp of ideal gas law concepts.
Visual Representation
The simulation provides a visual representation of the gas particles, allowing users to observe their movement and interactions. The molecules are color-coded to indicate their temperature, with warmer molecules appearing in brighter colors. Users can also observe the distribution of molecules within the container, with higher concentrations appearing as denser regions of color.
Real-time Data
The simulation displays real-time data on the temperature, volume, pressure, and number of moles of the gas. This data is updated dynamically as users make changes to the simulation parameters. The simulation also includes a graph that plots the relationship between pressure and volume, allowing users to visualize the behavior of the gas under different conditions.
Experiment Procedures
The Gizmo simulation provides a virtual laboratory environment to conduct experiments on the behavior of gases. Users can manipulate various variables and collect data to investigate the relationships between pressure, volume, temperature, and the number of gas particles.
Manipulating Variables
To manipulate variables in the Gizmo simulation:
- Pressure:Use the “Pressure” slider to adjust the pressure of the gas.
- Volume:Use the “Volume” slider to adjust the volume of the gas.
- Temperature:Use the “Temperature” slider to adjust the temperature of the gas.
- Number of particles:Use the “Particles” slider to adjust the number of gas particles.
Collecting Data
To collect data in the Gizmo simulation:
- Record the initial values:Before making any changes, record the initial values of pressure, volume, temperature, and the number of particles.
- Make a change:Manipulate one of the variables (e.g., pressure) and observe the changes in the other variables.
- Record the new values:After the changes have stabilized, record the new values of pressure, volume, temperature, and the number of particles.
- Repeat:Repeat steps 2 and 3 for different values of the manipulated variable.
Data Analysis
To analyze the data collected from the Gizmo simulation, follow these steps:
- Plot the data on a graph.
- Identify any trends in the data.
- Draw conclusions based on the trends.
Identifying Trends
When identifying trends in the data, look for patterns or relationships between the variables. For example, you might look for a linear relationship between pressure and volume, or an exponential relationship between temperature and volume.
Drawing Conclusions
Once you have identified the trends in the data, you can draw conclusions about the relationship between the variables. For example, if you find a linear relationship between pressure and volume, you can conclude that the pressure of a gas is directly proportional to its volume.
Applications of the Ideal Gas Law: Ideal Gas Law Gizmo Answers
The ideal gas law has numerous applications in various fields, including chemistry, engineering, and environmental science. It can be used to solve practical problems related to gas behavior and predict the behavior of gases under different conditions.
One common application is in the design and operation of internal combustion engines. The ideal gas law can be used to determine the amount of air and fuel needed for efficient combustion, optimizing engine performance and fuel efficiency.
Determining Gas Density, Ideal gas law gizmo answers
The ideal gas law can also be used to determine the density of a gas. Density is defined as mass per unit volume. By knowing the pressure, temperature, and molar mass of a gas, we can use the ideal gas law to calculate its density.
- For example, in the food industry, the ideal gas law is used to determine the density of carbon dioxide in carbonated beverages. This information is crucial for maintaining the correct level of carbonation and ensuring product quality.
Predicting Gas Behavior
Another important application is predicting the behavior of gases in chemical reactions. By knowing the initial conditions of a gas, such as pressure, volume, and temperature, the ideal gas law can be used to predict the final conditions after a reaction occurs.
- For instance, in the chemical industry, the ideal gas law is used to predict the yield of a chemical reaction. This information is essential for optimizing production processes and maximizing product yield.
FAQ Resource
What is the ideal gas law?
The ideal gas law is a mathematical equation that describes the relationship between the pressure, volume, temperature, and number of moles of a gas.
How can I use the Ideal Gas Law Gizmo?
The Ideal Gas Law Gizmo is a simulation that allows you to manipulate the variables of the ideal gas law and observe the effects on the gas.
What are some applications of the ideal gas law?
The ideal gas law can be used to solve a variety of problems, such as predicting the behavior of gases in industrial processes and understanding the dynamics of weather patterns.